November 2007: Enhanced Labelling of Neuromuscular Blocking Agents Makes a Difference
“Ideal features” for packaging and labelling of neuromuscular blocking agents were discussed during a collaborative meeting convened by ISMP Canada in 2006.† Since then, several manufacturers of these agents have incorporated some or all of the recommended features. ISMP Canada has received a report in which packaging features for a neuromuscular blocking agent helped to prevent a mix-up from reaching the patient:A nurse inadvertently selected a vial of the neuromuscular blocking agent succinylcholine (QUELICIN®, manufactured by Hospira) instead of the intended vial of heparin. As she was walking back to the patient’s bedside, she noticed white lettering on the red cap that read “WARNING: PARALYZING AGENT”. This prompted her to realize that she had selected the wrong vial. Incorrect administration and serious patient harm were thus averted.
ISMP Canada commends the manufacturers who have implemented the ideal features in their packaging and labelling of neuromuscular blockers and encourages all manufacturers to do so.†
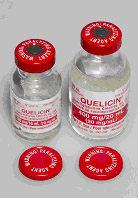 | Figure 1: Succinylcholine vials with a red cap, red ferrule with the words: “WARNING: PARALYZING AGENT”. |
†Neuromuscular blocking agent labelling and packaging initiative [Internet]. ISMP Can Safe Bull. 2006 [cited 2007 Oct 1];6(2):2. Available from: http://www.ismp-canada.org/download/ISMPCSB2006-02PotassiumPhosphates.pdf




