November 2009: Reported Look-Alike Concerns with Labelling for Sodium Chloride 0.9% and Hypertonic Sodium Chloride IV Solutions Lead to Change
ISMP Canada received a near-miss report where sodium chloride 5% was inadvertently used in a pharmacy for admixing a medication instead of the intended sodium chloride 0.9% (i.e., normal saline). Fortunately, the error was caught before the admixtures were dispensed.The reporter also identified a need to better differentiate the sodium chloride 3% product from the sodium chloride 0.9% product
(Figure 1).

Sodium chloride 0.9% is an isotonic solution that is frequently used for parenteral hydration, whereas sodium chloride 3% and 5% are hypertonic solutions used only for specific indications (e.g., the treatment of hyponatremia). The inadvertent administration of a hypertonic sodium chloride solution could lead to patient harm.
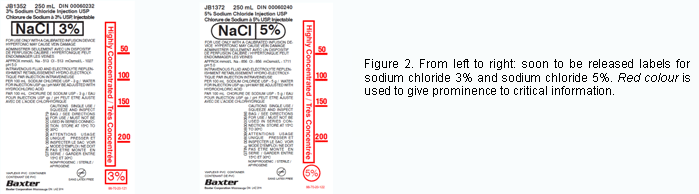
ISMP Canada notified Baxter Canada about the report. After consulting with its customers, Baxter Canada redesigned the labels for sodium chloride 3% and 5%, and sent copies of these soon-to-be-released labels to ISMP Canada (Figure 2).